

ラジカル反応によるジアステレオ選択的なホモアリルアルコールの合成
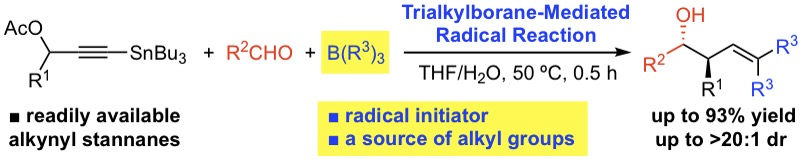
The trialkylborane/O2-mediated reaction of propargyl acetates having a tributylstannyl group at an alkyne terminus with aldehydes in a THF–H2O solvent system gave anti-δ,δ-disubstituted homoallylic alcohols with good to high diastereoselectivity. Intriguingly, two alkyl groups derived from trialkylborane were embedded into the reaction product. The trialkylborane plays a key role not only as a radical initiator but also as a source of alkyl radicals.
パラジウム触媒を用いた三成分連結反応よるアステレオ選択的なホモアリルアルコールの合成
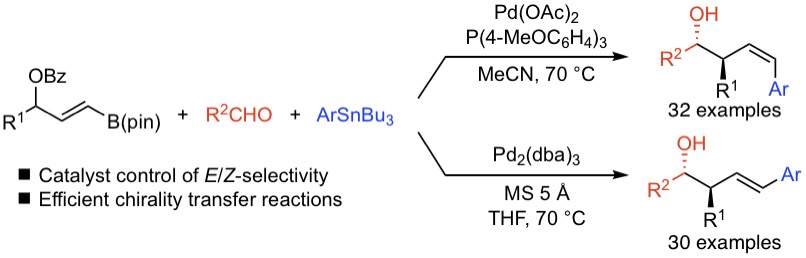
Diastereoselective synthesis of (Z)- and (E)-homoallylic alcohols using a Pd-catalyzed three-component reaction of 3-(pinacolatoboryl)allyl benzoates, aldehydes, and aryl stannanes was developed, which provides an alternative method for the allylboration of aldehydes using α,γ-diaryl-substituted allylboronates. Both sets of reaction conditions enable access to either (Z)- or (E)-homoallylic alcohols with good to high alkene stereocontrol. The present method showed good functional group compatibility and generality. Efficient chirality transfer reactions to afford enantioenriched (Z)- and (E)-homoallylic alcohols were also achieved.
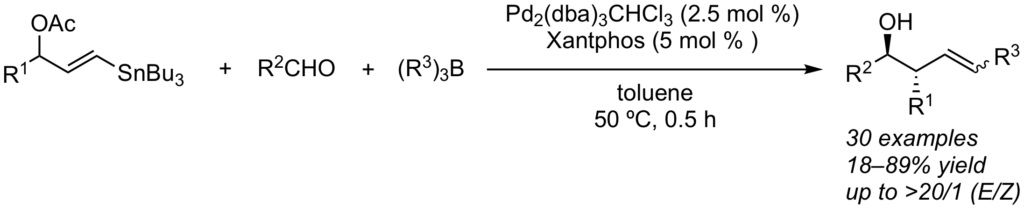
A three-component reaction of 3-(tri-n–butylstannyl)allyl acetates, aldehydes, and triorganoboranes in the presence of a palladium-Xantphos catalyst system predominately gave (E)-anti-homoallylic alcohols with high diastereoselectivity and good to high levels of alkene stereocontrol. An efficient chirality transfer was observed when an enantioenriched substrate was employed. The reaction was initiated by the formation of an allylic gem-palladium/stannyl intermediate, which subsequently underwent allylation of the aldehyde by an allyltributyltin followed by a coupling reaction of the in-situ-generated (E)-vinylpalladium acetate with the triorganoborane.
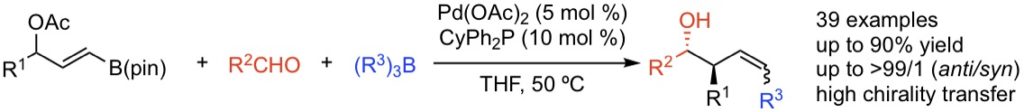
The Pd-catalyzed three-component reaction of 3-(pinacolatoboryl)allyl acetates, aldehydes, and organoboranes is described. The reaction is initiated by the formation of an allylic gem-palladium/boryl intermediate, which then undergoes allylation of aldehydes by allylboronates followed by a coupling reaction of in situ generated (Z)-vinylpalladium acetates with organoboranes to provide the (Z)-anti-homoallylic alcohols with high levels of diastereoselectivity and alkene stereocontrol.

堀野 良和准教授
HORINO Yoshikazu
略歴
専門分野